How to Approach Equilibrium Constant Problems in Chemistry Exams

Equilibrium constant problems are among the most challenging topics in chemistry exams, testing a student’s understanding of chemical reactions, equilibrium dynamics, and mathematical problem-solving. Whether you’re preparing for an online chemistry exam or facing a time-pressured test environment, mastering these problems is critical. Many students struggle with these questions and wonder, "Can someone take my chemistry exam?" With expert online exam help, such concerns can be alleviated. However, a strong theoretical grasp remains indispensable.
This blog delves into the theoretical foundation of equilibrium constant problems and provides a comprehensive guide on how to approach them effectively.
What is Chemical Equilibrium?
Before tackling equilibrium constant problems, it's essential to understand chemical equilibrium itself. In a reversible reaction, chemical equilibrium is the state where the forward and reverse reaction rates are equal, resulting in no net change in the concentration of reactants and products. However, this does not mean the reactions stop—molecular collisions and reactions continue dynamically.
Characteristics of Equilibrium:
- Dynamic Nature: The reaction doesn't cease; reactants are continuously converted into products and vice versa.
- Constant Concentrations: Once equilibrium is reached, the concentrations of all species remain constant, provided external conditions remain unchanged.
- Condition Dependency: Temperature, pressure, and concentration significantly influence the equilibrium state.
Equilibrium in Reactions
Let’s consider a generic reversible reaction:
aA+bB⇌cC+dD
Here:
- A and 𝐵 are reactants.
- C and 𝐷 are products.
- a,b,c, and 𝑑 are their respective stoichiometric coefficients.
At equilibrium, the concentrations of reactants and products follow the equilibrium constant expression.
What is the Equilibrium Constant?
The equilibrium constant (K) is a numerical value that indicates the ratio of products to reactants at equilibrium, raised to the power of their stoichiometric coefficients. This ratio provides insight into the extent of the reaction:
- If K>1: Products are favored; the reaction lies to the right.
- If K<1: Reactants are favored; the reaction lies to the left.
- If K=1: Reactants and products exist in comparable amounts.
Types of Equilibrium Constants:
- Kc: Based on molar concentrations (mol/L).
- Kp: Based on partial pressures (commonly used for gases).
The equilibrium constant expression is derived from the balanced chemical equation. For example, for the reaction:
aA+bB⇌cC+dD
The equilibrium constant (Kc) is expressed as:
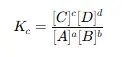
Where:
- [A],[B],[C],[Dare the equilibrium concentrations of each species.
- a,b,c,d are their stoichiometric coefficients.
Why Are Equilibrium Constant Problems Challenging?
Equilibrium constant problems demand the integration of multiple concepts:
- Balancing Reactions: An incorrect balanced equation leads to errors in the K expression.
- Mathematical Skills: Problems often involve solving quadratic equations or approximations.
- Conceptual Understanding: Knowing when and how to use Kc, Kp, or Q (reaction quotient).
In exam scenarios, time constraints further amplify these challenges. If you’ve thought, "Can someone take my exam for me?", it’s time to seek online exam help to reduce the burden while focusing on learning.
A Step-by-Step Guide to Solving Equilibrium Constant Problems
Step 1: Analyze the Given Reaction
- Write the balanced chemical equation.
- Identify if the problem involves Kc (concentration) or Kp (pressure).
- Determine whether the equilibrium concentrations are provided or need to be calculated.
Step 2: Identify Known and Unknown Values
- Extract given data: initial concentrations, equilibrium concentrations, or K.
- Use variables (x) to represent unknown changes.
Step 3: Write the Equilibrium Constant Expression
- Based on the balanced reaction, construct the KKK expression.
- For example:
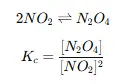
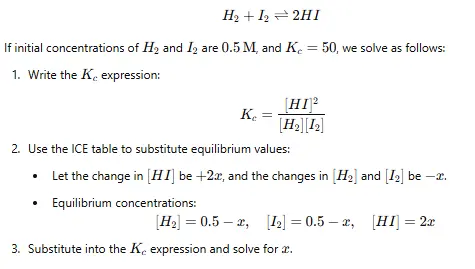
Step 4: Use the ICE Table Method
The ICE table organizes data systematically:
- I (Initial): Initial concentrations.
- C (Change): Changes during the reaction, often expressed as ±x.
- E (Equilibrium): Equilibrium concentrations, derived as Initial±Change.
For example, for the reaction:
Step 5: Solve for the Unknowns
- Simplify the equation algebraically.
- For quadratic equations, use the quadratic formula:
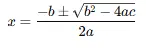
- Ensure your solution aligns with the initial conditions.
- Verify that concentrations are non-negative.
- Calculating 𝐾 from Equilibrium Data
In this problem type, equilibrium concentrations are provided, and you calculate 𝐾.

- Finding Equilibrium Concentrations
Here, you solve for the equilibrium concentrations using given 𝐾 and initial values.
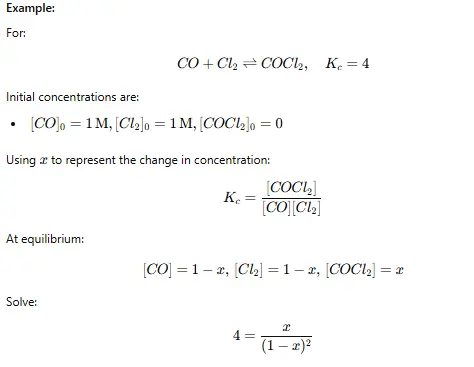
- Relationship Between Kc and Kp
For gaseous reactions, the relationship between Kc and Kp is:
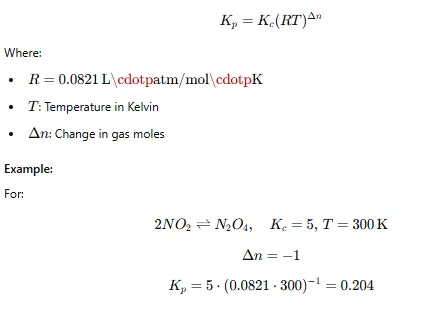
- Ignoring Stoichiometry: Incorrect coefficients lead to wrong K expressions.
- Misusing Q: Failing to distinguish Q (reaction quotient) from K.
- Rounding Errors: Premature rounding reduces accuracy.
Step 6: Verify Your Solution
Types of Equilibrium Constant Problems
Common Pitfalls in Equilibrium Problems
Conclusion
Equilibrium constant problems are manageable when approached methodically. By understanding chemical equilibrium, mastering Kc and Kp, and using the ICE table approach, you can confidently solve these problems. For those needing extra support, online exam help offers a lifeline, ensuring success in your chemistry exams. Reach out today and let expert assistance make a difference in your academic journey!